Flavonoids Extracts from Hunteria umbellata Seeds Modulates Streptozotocin-Induced Diabetes and Hepatic Complications in Rats
Main Article Content
Abstract
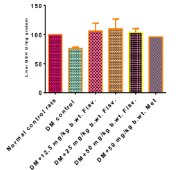
The use of flavonoid-rich plants has been previously reported for its potential in treating diabetes and its related complications. Synthetic medicines are commonly used to manage diabetes; however, they are costly and associated with various adverse effects. As a result, the search for a safer and affordable alternative from medicinal plants has become imperative to various complications caused by hyperglycaemia. Therefore, the present study aimed to investigate the modulatory effects of flavonoids from Hunteria Umbellata (H. umbellata) on hepatoxicity in streptozotocin-induced diabetic rats. A total of (60) male wistar rats (eight weeks old) weighing between 150-250grams were divided into six groups. Diabetes was induced in rats intraperitoneally with a single dose of streptozotocin (60 mg/kg body weight) while crude flavonoid extracts were administered orally into the rats with the following doses;12.5 mg/kg/bwt,25 mg/kg/bwt and 50 mg/kg/bwt. Assessment of apoptosis markers (Caspase-9, Bax, Bcl-2, cytochrome-c), lipid peroxidation (MDA), liver function tests (ALP, ALT, AST), antioxidant enzymes (SOD, CAT) and GSH were investigated. Histological changes of the liver tissues were also observed. In the in vivo antidiabetic experiments, the liver marker enzymes (ALP, ALT, AST) were significantly higher in the diabetic rats when compared with the control group (fig.1 to 5). The expression of the Caspase-9 gene, cytochrome c, and Bax in liver and kidney cells were significantly reduced in a dose-dependent manner when treated with 12.5 mg/kg, 25 mg/kg, and 50 mg/kg of crude flavonoids, as well as 50 mg/kg of metformin (Figures 6 and 7). The ameliorative effects of the crude flavonoids on the damaged liver tissues morphology were also observed. This study suggested that flavonoids from H. umbellata possesses ameliorative effects on liver apoptotic markers, lipid peroxidation, liver function marker enzymes and could be a potential alternative therapy for the management of diabetes and related complications.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
Oseni OA, Odesanmi EO, Oladele FC. Anti-oxidative and anti-diabetic activities of watermelon (Citrullus lanatus) juice on oxidative stress in alloxan-induced diabetic male Wistar albino rats. Niger Med J. 2015; 56(8):272-7. Doi: 10.4103/0300-1652.170382.
Kitada M, Ogura Y, Monno I, Koya D. Sirtuins and type 2 diabetes: role in inflammation, oxidative stress, and mitochondrial function. Front Endocrinol (Lausanne). 2019; 10(5):187. Doi: 10.3389/fendo.2019.00187.
Cuadros DF, Li J, Musuka G, Awad SF. Spatial epidemiology of diabetes: methods and insights. World J. Diab. 2021; 12(7):1042–56. Doi: 10.4239/wjd.v12.i7.1042.
Ojo OA, Akingbolabo DA, Ogunlakin D, Odesanmi EO, Ayomipo M, Berana G, Ayeni P, Ajayi-Odoko OA, Ayokunle DI, Ojo AB, Ajiboye BO, Ojo OO, Dahunsi SO. Preclinical antidiabetic and antioxidant effects of Erythrophleum africanum (Benth.) Harms in streptozotocin-induced diabetic nephropathy. J Complement Integr Med. 2024; 11(2): 157-171. Doi: 10.1515/jcim-2024-0090.
Gueguen N, Lenaers G, Reynier P, Weissig V, Edeas M. Mitochondrial dysfunction in mitochondrial medicine: current limitations, pitfalls, and tomorrow. Methods Mol Biol (Clifton, NJ). 2021; 2276(5):1–29. Doi: 10.1007/978-1-0716-1264-5_1.
Zhao X, Wang J, Deng Y, Liao L, Zhou M, Peng C. Quercetin as a protective agent for liver diseases: a comprehensive descriptive review of the molecular mechanism. Phytother Res. 2021; 35(9):4727–47. Doi: 10.1002/ptr.7167.
Okolie NP, Falodun A, Oluseyi D. Evaluation of the antioxidant activity of root extract of pepper fruit (Dennetia tripetala), and its potential for the inhibition of lipid peroxidation. Afr J Trad Compl Altern Med. 2014; 11(3):221-227. Doi: 10.4314/ajtcam.v11i3.31.
Dabeek WM, Marra MV. Dietary quercetin and kaempferol: bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients. 2019; 11(4):2288-2296. Doi: 10.3390/nu11102288.
Xu D, Hu MJ, Wang YQ, Cui YL. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules (Basel, Switzerland). 2019; 24(2):1123-1138. Doi: 10.3390/molecules24061123.
Tang SM, Deng XT, Zhou J, Li QP, Ge XX, Miao L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed Pharmacother. 2020; 121(3):109-604. Doi: 10.1016/j.biopha.2019.109604.
Hu Y, Gui Z, Zhou Y, Xia L, Lin K, Xu Y. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophage polarization to M2 macrophages. Free Radic Biol Med. 2019; 145(6):146–60. Doi: 10.1016/j.freeradbiomed.2019.10.002.
Han X, Xu T, Fang Q, Zhang H, Yue L, Hu G. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol. 2021; 44(5):102010-102024. Doi: 10.1016/j.redox.2021.102010.
Ojo OA, Akingbolabo DA, Ogunlakin D, Odesanmi EO, Ayomipo M, Berana G, Ayeni P, Ajayi-Odoko OA, Ayokunle DI, Ojo AB, Ajiboye BO, Ojo OO, Dahunsi SO. Preclinical antidiabetic and antioxidant effects of Erythrophleum africanum (Benth.) Harms in streptozotocin-induced diabetic nephropathy. J Complement Integr Med. 2024; 15(2): 307-318. Doi: 10.1515/jcim-2024-0090.
Albertini B, Sabatino M, Di Melegari C, Passerini N. Formulation of Spray Congealed Microparticles with Self-Emulsifying Ability for Enhanced Glibenclamide Dissolution Performance.J.Microencapsul.2015;32(2):181–192.Doi:10.3109/02652048.2014.991327.
Boccellino M, D'Angelo S. Anti-obesity effects of polyphenol intake: current status and future possibilities. Int J Mol Sci. 2020; 21(16):5642. Doi: 10.3390/ijms21165642.
Adefegha SA, Oboh G. Enhancement of total phenolics and antioxidants properties of tropical green leafy vegetables by steam cooking. J Food Process Preserv. 2011; 35(5):615-622. Doi: 10.1111/j.1745-4549.2010.00404.x.
Chu Y, Sun J, Wu X, Liu RH. Antioxidant and antiproliferation activity of common vegetables. J Agric Food Chem. 2002; 50(23):6910–6916. Doi: 10.1021/jf020665f.
Musabayane CT, Mahlalela N, Shode FO, Ojewole JAO. Effects of Syzygium cordatum (Hochst.) [Myrtaceae] leaf extract on plasma glucose and hepatic glycogen in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2005; 97(3):485-490. Doi: 10.1016/j.jep.2004.11.027.
Ogunmoyole T, Folashade TA, John I A, Odesanmi EO, Jimoh T. Calotropis procera leaf extract ameliorates oxidant induced hepatic and renal damage: potential relevance in the management of liver and kidney diseases. Medplant-Inter J Phytome. 2023; 15 (2): 307-318. Doi.10.5958/0975-6892.2023.00030.
Reitman S, Frankel SA. colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957; 28(1):56-63. Doi: 10.1093/ajcp/28.1.56.
Wright PJ, Leathwood PD, Plummer DT. Enzyme activity in human plasma and serum: the effect of specimen collection and handling. Clin Chim Acta. 1972; 37(1):193-197. Doi: 10.1016/0009-8981(72)90437-9.
Misra HP, Fridovich I. The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972; 247(10):3170-3175. Doi: 10.1016/S0021-9258(19)45228-9.
Rodrigues NR, Rowan A, Smith M, Kerr IB, Bodmer WF, Gannon JV. p53 mutations in colorectal cancer. Proc Natl Acad Sci U S A. 1990; 87(19):7555-7559. Doi: 10.1073/pnas.87.19.7555.
Disbrey AT, Rack EP. A standardized method for histological preparation of the human placenta for morphometric studies. Placenta. 1970; 1(1):21-35. Doi: 10.1016/S0143-4004(70)80007-7.
Cheung PY, Klotz U. Clinical pharmacokinetics of magnesium sulfate. Clin Pharmacokinet. 1997; 32(2):146-160. Doi: 10.2165/00003088-199732020-00003.
Kapoor R, Srivastava S, Kakkar P. Bacopa monnieri modulates antioxidant responses in brain and kidney of diabetic rats. Environ Toxicol Pharmacol. 2009; 27(1):62–69. Doi: 10.1016/j.etap.2008.07.002.
Zhao X, Wang J, Deng Y, Liao L, Zhou M, Peng C. Quercetin as a protective agent for liver diseases: a comprehensive descriptive review of the molecular mechanism. Phytother Res. 2021; 35(9):4727–4747. Doi: 10.1002/ptr.7130.
Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009; 48(1):44–51. Doi: 10.1016/j.plipres.2008.10.002.
Dabeek WM, Marra MV. Dietary quercetin and kaempferol: bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients. 2019; 11(10):2288. Doi: 10.3390/nu11102288.
Bell DS, Allbright E. The multifaceted associations of hepatobiliary disease and diabetes. Endocr Pract. 2007; 13(3):300–312. Doi: 10.4158/EP.13.3.300.
Kitada M, Ogura Y, Monno I, Koya D. Sirtuins and type 2 diabetes: role in inflammation, oxidative stress, and mitochondrial function. Front Endocrinol (Lausanne). 2019; 10(3):187. Doi: 10.3389/fendo.2019.00187.
Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993; 215(2):213-219. Doi: 10.1111/j.1432-1033.1993.tb18025.x.
Han X, Xu T, Fang Q, Zhang H, Yue L, Hu G. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol. 2021; 44:102010-102025. Doi: 10.1016/j.redox.2021.102010.
Jubaidi FF, Zainalabidin S, Taib IS, Hamid ZA, Budin SB. The potential role of flavonoids in ameliorating diabetic cardiomyopathy via alleviation of cardiac oxidative stress, inflammation, and apoptosis. Int J Mol Sci. 2021; 22(10):5094-5093. Doi: 10.3390/ijms22105094.
Wang JN, Yang Q, Yang C, Cai YT, Xing T, Gao L. Smad3 promotes AKI sensitivity in diabetic mice via interaction with p53 and induction of NOX4-dependent ROS production. Redox Biol. 2020; 32(6):101479-101494. Doi: 10.1016/j.redox.2020.101479.
Ye F, Wu A. The protective mechanism of SIRT1 in the regulation of mitochondrial biogenesis and mitochondrial autophagy in Alzheimer's disease. J Alzheimer's Dis. 2021; 82(1):149–157. Doi: 10.3233/JAD-201013.
Xie C, Wu W, Tang A, Luo N, Tan Y. lncRNA GAS5/miR-452-5p reduces oxidative stress and pyroptosis of high-glucose-stimulated renal tubular cells. Diabetes Metab Syndr Obes. 2019; 12(4):2609–2617. Doi: 10.2147/DMSO.S217095.
Pena-Blanco A, Garcia-Saez AJ. Bax, Bak and beyond–mitochondrial performance in apoptosis. FEBS J. 2018; 285(6):416–431. Doi: 10.1111/febs.14186.
Shao Y-X, Xu X-X, Wang K, Qi X-M, Wu Y-G. Paeoniflorin attenuates incipient diabetic nephropathy in streptozotocin-induced mice by the suppression of the Toll-like receptor-2 signaling pathway. Drug Des Dev Ther. 2017; 11(2):3221–3233. Doi: 10.2147/DDDT.S150936.
Wang JN, Yang Q, Yang C, Cai YT, Xing T, Gao L. Smad3 promotes AKI sensitivity in diabetic mice via interaction with p53 and induction of NOX4-dependent ROS production. Redox Biol. 2020; 32(5):101479-101492. Doi: 10.1016/j.redox.2020.101479.
Du XS, Li HD, Yang XJ, Li JJ, Xu JJ, Chen Y. Wogonin attenuates liver fibrosis via regulating hepatic stellate cell activation and apoptosis. Int Immunopharmacol. 2019; 75(6):105671. Doi: 10.1016/j.intimp.2019.105671.
Hong M, Almutairi MM, Li S, Li J. Wogonin inhibits cell cycle progression by activating the glycogen synthase kinase-3 beta in hepatocellular carcinoma. Phytomed J. 2020; 68(6):153174. Doi: 10.1016/j.phymed.2019.153174.
Gui X, Yang H, Li T, Tan X, Shi P, Li M. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature. 2019; 567(7):262–266. Doi: 10.1038/s41586-019-1006-9.
Pena-Blanco A, Garcia-Saez AJ. Bax, Bak and beyond–mitochondrial performance in apoptosis. Fed Eeur Bio Soc J. 2018; 285(5):416–431. Doi: 10.1111/febs.14186.