Characterization of Immobilized Endo-Polygalacturonase PGC-AN64 from Aspergillus niger HO32 with Potential Biotechnological Interest
Main Article Content
Abstract
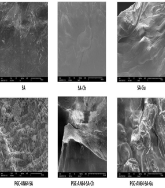
This study focuses on the characterization and application of an immobilized purified endo-polygalacturonase PGC-AN64 from Aspergillus niger HO32 using sodium alginate (SA), and its combinations with guar gum (SA-Gu) or chitosan (SA-Ch) as supports. Immobilization efficiencies were 75%, 83%, and 77% for PGC-AN64-SA, PGC-AN64-SA-Ch, and PGC-AN64-SA-Gu, respectively. Immobilized enzymes showed enhanced thermostability and pH stability. At 80°C, the half-lives reached 8, 11, and 16 h, and residual activity at pH 5 after extended incubation (12–24 h) was maintained at approximately 56–58%, depending on the matrix. Reusability tests showed activity retention of ~50% for PGC-AN64-SA after 4 cycles, and ~64% and 51% for PGC-AN64-SA-Ch and PGC-AN64-SA-Gu, respectively, after 6 cycles. Orange juice clarification efficiency was reflected by transmittance values of 85.20±1.68% for free PGC-AN64, and ~84% for all immobilized forms. XRD, FTIR-ATR, and FE-SEM analyses confirmed structural changes upon immobilization. In addition, orange juice clarification resulted in a decrease in total soluble solids and color modifications: reduced a* values, and increased L* and b* values. These findings highlight that immobilizing PGC-AN64 on SA-based matrices improves enzyme stability, operational reusability, and retains orange juice clarification performance. The approach offers an efficient and safe enzymatic solution for agro-industrial applications, particularly in juice processing.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1 Zafar T, Hadri S, Imran M, Asad J, Athar I, Mahmood R. Production, purification and characterization of endo-polygalacturonase using novel strain of Bacillus pumilus through RSM. Process Biochem. 2024; 146:188–194. doi.org/10.1016/j.procbio.2024.07.029
2 Zhong L, Wang X, Fan L, Ye X, Li Z, Cui Z, Huang Y. Characterization of an acidic pectin methylesterase from Paenibacillus xylanexedens and its application in fruit processing. Protein Expres Purif. 2021; 179:105798. doi.org/10.1016/j.pep.2020.105798
3 Rezaei PF, Ghanbari S, Mahdavinia G. Purification and immobilization of exo-polygalacturonase using nanomagnetic beads. Process Biochem. 2023; 132:180–190. doi.org/10.1016/j.procbio.2023.06.018
4 Satapathy S, Rout JR, Kerry RG, Thatoi H, Sahoo SL. Biochemical prospects of various microbial pectinase and pectin: an approachable concept in pharmaceutical bioprocessing. Front Nutr. 2020; 7:117. doi.org/10.3389/fnut.2020.00117
5 Arpana M, Rathore SS, Rao AS, Nair A, More SS, Fasim A. Statistical bioprocess optimization for enhanced production of a thermo alkalophilic polygalacturonase (PGase) from Pseudomonas sp. using solid substrate fermentation (SSF). Heliyon. 2023; 9:e16493. doi.org/10.1016/j.heliyon.2023.e16493
6 Jahan N, Shahid F, Aman A, Mujahid TY, Qader SAU. Utilization of agro waste pectin for the production of industrially important polygalacturonase. Heliyon. 2017; 3:03-30. doi.org:doi:10.1016/j.heliyon.2017.e00330
7 Núñez-Serrano A, García-Reyes RB, Solís-Pereira S, García-González A. Production and immobilization of pectinases from Penicillium crustosum in magnetic core-shell nanostructures for juice clarification. Int J Biol Macromol. 2024; 263:130268. doi.org/10.1016/j.ijbiomac.2024.130268
8 John J, Kaimal KS, Smith ML, Rahman PK, Chellam PV. Advances in upstream and downstream strategies of pectinase bioprocessing: a review. Int J Biol Macromol. 2020; 162:1086–1099. doi.org/10.1016/j.ijbiomac.2020.06.224
9 Blanco P, Sieiro C, Villa TG. Production of pectic enzymes in yeasts. FEMS Microbiol Lett. 1999; 175:1–9. doi.org/10.1111/j.1574-6968.1999.tb13595.x
10 Reginatto C, Rossi C, Miglioranza BG, dos Santos M, Meneghel L, da Silveira MM, Malvessi E. Pectinase production by Aspergillus niger LB-02-SF is influenced by the culture medium composition and the addition of the enzyme inducer after biomass growth. Process Biochem. 2017; 58:1–8. doi.org/10.1016/j.procbio.2017.04.018
11 Kant S, Vohra A, Gupta R. Purification and physicochemical properties of polygalacturonase from Aspergillus niger MTCC 3323. Protein Expr Purif. 2013; 87:11-16. doi.org/10.1016/j.pep.2012.09.014
12 He Y, Pan L, Wang B. Efficient over-expression and application of high-performance pectin lyase by screening Aspergillus niger pectin lyase gene family. Biotechnol Bioprocess Eng. 2018; 23:662-669. doi.org/10.1007/s12257-018-0387-1
13 Barman S, Sit N, Badwaik LS, Deka SC. Pectinase production by Aspergillus niger using banana (Musa balbisiana) peel as substrate and its effect on clarification of banana juice. J Food Sci Technol. 2015; 52:3579-3589. doi.org/10.1007/s13197-014-1413-8
14 Bentouhami NE, Asehraou A, Mechri S, Hasnaoui I, Moumnassi S, Yahyaoui MI, Jaouadi B. Purification and biochemical characterization of a novel thermostable endo-polygalacturonase from Aspergillus niger strain HO32 and its suitability for clarification of orange juice. Process Biochem. 2024; 145:63-73. doi.org/10.1016/j.procbio.2024.06.013
15 Kaul K, Rajauria G, Singh R. Valorization of agro-industrial waste for pectinase production and its influence on circular economy. Food Bioprod Process. 2024; 148:141-153. doi.org/10.1016/j.fbp.2024.09.008
16 Mahmoodi M, Najafpour G, Mohammadi M. Bioconversion of agroindustrial wastes to pectinases enzyme via solid state fermentation in trays and rotating drum bioreactors. Biocatal Agric Biotechnol. 2019; 21:101280. doi.org/10.1016/j.bcab.2019.101280
17 Martínez-Trujillo A, Arreguín-Rangel L, García-Rivero M, Aguilar-Osorio G. Use of fruit residues for pectinase production by Aspergillus flavipes FP-500 and Aspergillus terreus FP-370. Lett Appl Microbiol. 2011; 53:202-209. doi.org/10.1111/j.1472-765X.2011.03096.x
18 Li J, Yang M, Zhao F, Zhang Y, Han S. Efficient expression of an alkaline pectin lyase from Bacillus licheniformis in Pichia pastoris. Bioresour Bioprocess. 2024; 11:37. doi.org/10.1186/s40643-024-00752-w
19 Cheng Z, Chen D, Wang Q, Xian L, Lu B, Wei Y, Huang R. Identification of an acidic endo-polygalacturonase from Penicillium oxalicum CZ1028 and its broad use in major tropical and subtropical fruit juices production. J Biosci Bioeng. 2017; 123:665-672. doi.org/10.1016/j.jbiosc.2017.01.013
20 Nwokeoma CI, Arotupin DJ, Olaniyi OO, Adetuyi FC. Enhanced production, purification and characterization of pectinase from Aspergillus flavus. Trop J Nat Prod Res. 2021; 5:1876-1882. doi.org/10.26538/tjnpr/v5i10.28
21 Sharma N, Patel SN, Rai AK, Singh SP. Biochemical characterization of a novel acid-active endopolygalacturonase for pectin depolymerization, pectic-oligomer production, and fruit juice clarification. Int J Biol Macromol. 2024; 267:131565. doi.org/10.1016/j.ijbiomac.2024.131565
22 Carli S, Meleiro LP, Ward RJ. Biochemical and kinetic characterization of the recombinant GH28 Stereum purpureum endopolygalacturonase and its biotechnological application. Int J Biol Macromol. 2019; 137:469-474. doi.org/10.1016/j.ijbiomac.2019.06.165
23 Sandri IG, Fontana RC, Barfknecht DM, da Silveira MM. Clarification of fruit juices by fungal pectinases. LWT-Food Sci Technol. 2011; 44:2217-2222. doi.org/10.1016/j.lwt.2011.02.008
24 Carli S, de Campos Carneiro LAB, Ward RJ, Meleiro LP. Immobilization of a β-glucosidase and an endoglucanase in ferromagnetic nanoparticles: A study of synergistic effects. Protein Expr Purif. 2019; 160:28-35. doi.org/10.1016/j.pep.2019.03.016
25 Ji L, Chen M, Zhang W, Nian B, Hu Y. Comprehensive applications of ionic liquids in enzyme immobilization: Current status and prospects. Mol Catal. 2024; 552:113675. doi.org/10.1016/j.mcat.2023.113675
26 de Oliveira RL, Dias JL, da Silva OS, Porto TS. Immobilization of pectinase from Aspergillus aculeatus in alginate beads and clarification of apple and umbu juices in a packed bed reactor. Food Bioprod Process. 2018; 109:9-18. doi.org/10.1016/j.fbp.2018.02.005
27 Ramirez HL, Gómez Brizuela L, Úbeda Iranzo J, Arevalo-Villena M, Briones Pérez AI. Pectinase immobilization on a chitosan-coated chitin support. J Food Process Eng. 2016; 39:97-104. doi.org/10.1111/jfpe.12203
28 Kaushal J, Khatri M, Singh G, Arya SK. Xylanase enzyme from novel strain and its immobilization onto metal-organic framework MOF for fruit juice clarification. Biotechnol Bioprocess Eng. 2024; 29:197-210. doi.org/10.1007/s12257-024-00007-7
29 Wang B, Cheng F, Lu Y, Ge W, Zhang M, Yue B. Immobilization of pectinase from Penicillium oxalicum F67 onto magnetic cornstarch microspheres: Characterization and application in juice production. J Mol Catal B: Enzym. 2013; 97:137-143. doi.org/10.1016/j.molcatb.2013.07.018
30 Navarro-López DE, Bautista-Ayala AR, Rosales-De la Cruz MF, Martínez-Beltrán S, Rojas-Torres DE, Sanchez-Martinez A, López-Mena ER. Nanocatalytic performance of pectinase immobilized over in situ prepared magnetic nanoparticles. Heliyon. 2023; 9:e19-21. doi.org/10.1016/j.heliyon.2023.e19021
31 Rajdeo K, Harini T, Lavanya K, Fadnavis NW. Immobilization of pectinase on reusable polymer support for clarification of apple juice. Food Bioprod Process. 2016; 99:12-19. doi.org/10.1016/j.fbp.2016.03.004
32 Chen M, She W, Zhao X, Chen C, Zhu B, Sun Y, Yao Z. Immobilization of Thermomyces lanuginosus lipase in a novel polysaccharide-based hydrogel by a two-step crosslinking method and its use in the lauroylation of α-arbutin. Bioresour Bioprocess. 2024; 11:7. doi.org/10.1186/s40643-023-00721-9
33 Xie P, Lan J, Zhou J, Hu Z, Cui J, Qu G, Sun Z. Co-immobilization of amine dehydrogenase and glucose dehydrogenase for the biosynthesis of (S)-2-aminobutan-1-ol in continuous flow. Bioresour Bioprocess. 2024; 11:70-72. doi.org/10.1186/s40643-024-00786-0
34 Wei C, Zhou Y, Zhuang W, Li G, Jiang M, Zhang H. Improving the performance of immobilized β-glucosidase using a microreactor. J Biosci Bioeng. 2018; 125:377-384. doi.org/10.1016/j.jbiosc.2017.09.011
35 Amin F, Bhatti HN, Bilal M, Asgher M. Improvement of activity, thermo-stability and fruit juice clarification characteristics of fungal exo-polygalacturonase. Int J Biol Macromol. 2017; 95:974-984. doi.org/10.1016/j.ijbiomac.2016.10.086
36 Mechri S, Allala F, Bouacem K, Hasnaoui I, Gwaithan H, Chalbi TB, Jaouadi B. Preparation, characterization, immobilization, and molecular docking analysis of a novel detergent-stable subtilisin-like serine protease from Streptomyces mutabilis strain TN-X30. Int J Biol Macromol. 2022; 222:1326-1342. doi.org/10.1016/j.ijbiomac.2022.09.161
37 Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959; 31:426-428. doi.org/10.1021/ac60147a030
38 Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248-254 doi.org/10.1016/0003-2697(76)90527-3
39 Ellerbrock RH, Gerke HH. FTIR spectral band shifts explained by OM–cation interactions. J Plant Nutr Soil Sci. 2021; 184:388-397. doi.org/10.1002/jpln.202100056
40 Xu H, Cao Y, Li X, Cao X, Xu Y, Qiao D, Cao Y. An electrospun chitosan-based nanofibrous membrane reactor immobilized by the non-glycosylated rPGA for hydrolysis of pectin-rich biomass. Renew Energy. 2018; 126:573-582. doi.org/10.1016/j.renene.2017.08.094
41 Almulaiky YQ, Alkabli J, El-Shishtawy RM. Improving enzyme immobilization: A new carrier-based magnetic polymer for enhanced covalent binding of laccase enzyme. Int J Biol Macromol. 2024; 8:137-362.
42 Wu Y, Meng F, Fu J, Zhou Y, Fan H, Ju X, Li L. PDA/SiO2 microspheres immobilize dual enzymes to increase temperature tolerance and catalyze the production of D-ribulose and D-xylulose from D-xylose. Mol Catal. 2024; 555:113-900. doi.org/10.1016/j.mcat.2024.113900
43 Ellerbrock R, Höhn A, Rogasik J. Functional analysis of soil organic matter as affected by long‐term manurial treatment. Eur J Soil Sci. 1999; 50:65-71. doi.org/10.1046/j.1365-2389.1999.00206.x
44 Hosseini SS, Khodaiyan F, Mousavi SM, Azimi SZ. Clarification of the pomegranate juice in a bioreactor packed by pectinase enzymes immobilized on the glass bead activated with polyaldehyde polysaccharides. LWT. 2021; 137:110500. doi.org/10.1016/j.lwt.2020.110500
45 Rehman HU, Aman A, Silipo A, Qader SAU, Molinaro A, Ansari A. Degradation of complex carbohydrate: immobilization of pectinase from Bacillus licheniformis KIBGE-IB21 using calcium alginate as a support. Food Chem. 2013; 139:1081-1086. doi.org/10.1016/j.foodchem.2013.01.069
46 Ramirez HL, Briones AI, Úbeda J, Arevalo M. Immobilization of pectinase by adsorption on an alginate-coated chitin support. Biotecnol Apl. 2013; 30:101-104.
47 Xue L, Long J, Lu C, Li X, Xu X, Jin Z. Immobilization of polygalacturonase for the preparation of pectic oligosaccharides from mango peel wastes and assessment of their antibacterial activities. Food Biosci. 2021; 39:100837. doi.org/10.1016/j.fbio.2020.100837
48 Busto M, García-Tramontín K, Ortega N, Perez-Mateos M. Preparation and properties of an immobilized pectinlyase for the treatment of fruit juices. Bioresour Technol. 2006; 97:1477-1483. doi.org/10.1016/j.biortech.2005.06.013
49 Sharma G, Sharma S, Kumar A, Al-Muhtaseb AAH, Naushad M, Ghfar AA, Stadler FJ. Guar gum and its composites as potential materials for diverse applications: A review. Carbohydr Polym. 2018; 199:534-545.
50 Gülay S, Şanlı-Mohamed G. Immobilization of thermoalkalophilic recombinant esterase enzyme by entrapment in silicate coated Ca-alginate beads and its hydrolytic properties. Int J Biol Macromol. 2012; 50:545-551. doi.org/10.1016/j.ijbiomac.2012.01.017
51 Karataş E, Tülek A, Yildirim D, Tamtürk F, Binay B. Immobilization of Sporothrix schenckii 1099-18 exo-polygalacturonase in magnetic mesoporous silica yolk-shell spheres: Highly reusable biocatalysts for apple juice clarification. Food Biosci. 2021; 43:101324. doi.org/10.1016/j.fbio.2021.101324
52 Tapdigov SZ. The bonding nature of the chemical interaction between trypsin and chitosan based carriers in immobilization process depend on entrapped method: A review. Int J Biol Macromol. 2021; 183:1676-1696. doi.org/10.1016/j.ijbiomac.2021.05.059
53 Işik C, Arabaci G, Doğaç YI, Deveci İ, Teke MS. Synthesis and characterization of electrospun PVA/Zn2+ metal composite nanofibers for lipase immobilization with effective thermal, pH stabilities and reusability. Mater Sci Eng C. 2019; 99:1226-1235. doi.org/10.1016/j.msec.2019.02.031
54 Carli S, Salgado JCS, Meleiro LP, Ward RJ. Covalent immobilization of Chondrostereum purpureum endopolygalacturonase on ferromagnetic nanoparticles: catalytic properties and biotechnological application. Appl Biochem Biotechnol. 2022; 12:1-14. doi.org/10.1007/s12010-021-03688-5
55 Rehman HU, Nawaz MA, Pervez S, Jamal M, Attaullah M, Aman A, Qader SAU. Encapsulation of pectinase within polyacrylamide gel: Characterization of its catalytic properties for continuous industrial uses. Heliyon. 2020; 6:45-78. doi.org/10.1016/j.heliyon.2020.e04578
56 Oktay B, Demir S, Kayaman-Apohan N. Immobilization of pectinase on polyethyleneimine based support via spontaneous amino-yne click reaction. Food Bioprod Process. 2020; 122:159-168. doi.org/10.1016/j.fbp.2020.04.010
57 dos Santos EG, de Assis SA, Ferreira AN, de Almeida Bezerra M, de Paula SA, Valasques LM, Júnior GLV. Production, characterization and application of polygalacturonase produced by Lentinus tigrinus CCMB 553. Biocatal Agric Biotechnol. 2024; 58:103216. doi.org/10.1016/j.bcab.2024.103216
58 Gani G, Naik HR, Jan N, Bashir O, Hussain SZ, Rather AH, Amin T. Physicochemical and antioxidant properties of pear juice prepared through pectinase enzyme-assisted extraction from William Bartlett variety. J Food Meas Charact. 2021; 15:743-757. doi.org/10.1007/s11694-020-00676-x
59 Diano N, Grimaldi T, Bianco M, Rossi S, Gabrovska K, Yordanova G, Mita DG. Apple juice clarification by immobilized pectolytic enzymes in packed or fluidized bed reactors. J Agric Food Chem. 2008; 56:11471-11477. doi.org/10.1021/jf8019437