Enhancing analgesic and anti-inflammatory synergetic effect with Naringenin: In-silico and in vivo investigation
Main Article Content
Abstract
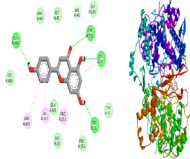
Chronic inflammation and pain are major clinical challenges, often requiring pharmacological intervention. While NSAIDs like Aceclofenac and opioid analgesics such as Pentazocine are effective, their long-term use is associated with adverse effects. Naringenin, a natural bioactive compound, has demonstrated promising anti-inflammatory and analgesic properties, suggesting potential for combination therapy to enhance efficacy and reduce side effects. This study investigates the synergistic effects of Naringenin with Aceclofenac and Pentazocine through molecular docking and in vivo animal models. Molecular docking revealed strong binding interactions of Naringenin with COX-2 (-9.1 kcal/mol), μ-opioid receptors (-7.8 kcal/mol), and TNF-α (-7.6 kcal/mol), indicating potential analgesic and anti-inflammatory activity. Acetic acid-induced writhing test (peripheral analgesic activity) effects showed highly significant inhibition (***p<0.001) at both high (21.83 ± 1.276, 100 mg/kg, p.o.) and low (23.33 ± 0.881, 50 mg/kg, p.o.) doses of Aceclofenac combined with Naringenin (200 mg/kg, p.o.). The hotplate test (central analgesic activity) revealed a highly significant response (***p<0.001) after 30 min at high (12.33±0.494, 10 mg/kg, iv.) and low (11.00±0.365, 5 mg/kg, iv.) doses of Pentazocine with Naringenin. Carrageenan-induced paw edema (anti-inflammatory effect) showed significant inhibition (***p<0.001) at similar dose combinations after 1 hr (high dose, 0.422±0.020 and low dose, 0.444±0.015) Additionally, inflammatory biomarkers CRP and TNF-α exhibited highly significant reductions (***p<0.001), while IL-6 (showed significant reduction (**p<0.01). These findings suggest that Naringenin enhances the therapeutic efficacy of Aceclofenac and Pentazocine, offering a potential combinatorial strategy for safer and more effective pain and inflammation management.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
Baral P, Udit S, Chiu IM. Pain and immunity: Implications for host defence. Nat Rev Immunol. 2019; 19(7):433–447. Doi: 10.1038/s41577-019-0147-2
Zobdeh F, Eremenko II, Akan MA, Tarasov VV, Chubarev VN, Schiöth HB, Mwinyi J. Pharmacogenetics and pain treatment with a focus on non-steroidal anti-inflammatory drugs (NSAIDs) and antidepressants: A systematic review. Pharmaceut. 2022; 14(6):1190. Doi: 10.3390/pharmaceutics14061190
Paul B, Sribhashyam S, Majumdar S. Opioid signaling and design of analgesics. Prog Mol Biol Transl Sci. 2023; 195:153–176. Doi: 10.1016/bs.pmbts.2022.06.017
Coluzzi F. Caputi FF, Billeci D, Pastore AL, Candeletti S, Rocco M, Romualdi P. Safe use of opioids in chronic kidney disease and hemodialysis patients: Tips and tricks for non-pain specialists. Ther Clin Risk Manag. 2020; 16:821–837. Doi: 10.2147/TCRM.S262843
Shilpa VS, Shams R, Dash KK, Pandey VK, Dar AH, Ayaz Mukarram S, Harsányi E, Kovács B. Phytochemical properties, extraction, and pharmacological benefits of naringin: A review. Molec. 2023; 28(15):5623.
Al-Dabbagh MA, Sahib HB. Naringenin ameliorates lipopolysaccharide-induced kidney and lung injuries in mice: Decisive role of IL-1β, IL-6, IL-8, and TNF-α. Opera Med Physiol. 2024; 11(1):34–51.
Chen R, Coppes M, Urman RD. Receptor and molecular targets for the development of novel opioid and non-opioid analgesic therapies. Pain Physician. 2021; 24(2):153.
Jabir NR, Shakil S, Tabrez S, Khan MS, Rehman MT, Ahmed BA. In silico screening of glycogen synthase kinase-3β targeted ligands against acetylcholinesterase and its probable relevance to Alzheimer’s disease. J Biomol Struct Dyn. 2021; 39(14):5083–5092. Doi: 10.1080/07391102.2020.1784796
Baek SH, Hwang S, Park T, Kwon YJ, Cho M, Park D. Evaluation of selective COX-2 inhibition and in silico study of kuwanon derivatives isolated from Morus alba. Int J Mol Sci. 2021; 22(7):3659. Doi:10.3390/ijms22073659
Alananzeh WA, Al-Qattan MN, Ayipo YO, Mordi MN. N-substituted tetrahydro-beta-carboline as mu-opioid receptors ligands: In silico study; molecular docking, ADMET and molecular dynamics approach. Mol Divers. 2024; 28(3):1273–1289. Doi:10.1007/s11030-023-10655-1
Agnihotri P, Deka H, Chakraborty D, Monu N, Saquib M, Kumar U, Biswas S. Anti-inflammatory potential of selective small compounds by targeting TNF-α & NF-κB signaling: A comprehensive molecular docking and simulation study. J Biomol Struct Dyn. 2023; 41(23):13815–13828. Doi:10.1080/07391102.2023.2196692
Dhamak VM, Amrutkar SV. Nephroprotective effects of Spirulina platensis on NRK-52E cell line: LC-HRMS and docking studies targeting epidermal growth factor receptor. Trop J Nat Prod Res. 2023; 7(7):1053–1058.
Edoga CO, Anukwuorji CA. Effect of vitamin E on serum albumin, total protein, total and conjugated bilirubin of male Wistar albino rats infected with Trypanosoma brucei. Glob Sci J. 2021; 9(10):118-131.
Safhi MM. Nephroprotective effect of zingerone against CCl₄-induced renal toxicity in Swiss albino mice: Molecular mechanism. Oxid Med Cell Longev. 2018; 2474831.
Santenna C, Kumar S, Balakrishnan S, Jhaj R, Ahmed SN. A comparative experimental study of analgesic activity of a novel non-steroidal anti-inflammatory molecule–zaltoprofen, and a standard drug–piroxicam, using murine models. J Exp Pharmacol. 2019; 11:85–91. Doi: 10.2147/JEP.S212988
Parwani L, Bhatnagar M, Bhatnagar A, Sharma V, Sharma V. Gum acacia-PVA hydrogel blends for wound healing. Vegetos. 2019; 32(1):78–91. Doi: 10.1007/s42535-019-00009-4
Lee YM, Son E, Kim SH, Kim DS. Anti-inflammatory and analgesic effects of Schisandra chinensis leaf extracts and monosodium iodoacetate-induced osteoarthritis in rats and acetic acid-induced writhing in mice. Nutrients. 2022; 14(7):1356. Doi: 10.3390/nu14071356
Hu CY, Zhao YT. Analgesic effects of naringenin in rats with spinal nerve ligation-induced neuropathic pain. Biomed Rep. 2014; 2(4):569-573. doi: 10.3892/br.2014.267.
Jo HG, Lee GY, Baek CY, Song HS, Lee D. Analgesic and anti-inflammatory effects of Aucklandia lappa root extracts on acetic acid-induced writhing in mice and monosodium iodoacetate-induced osteoarthritis in rats. Plan. 2020; 10(1):42. Doi:10.3390/plants10010042
Nakhaee S, Dastjerdi M, Roumi H, Mehrpour O, Farrokhfall K. N-acetylcysteine dose-dependently improves the analgesic effect of acetaminophen on the rat hot plate test. BMC Pharmacol Toxicol. 2021; 22:1–7. Doi: 10.1186/s40360-020-00469-4
Modi AD, Parekh A, Pancholi YN. Evaluating pain behaviours: Widely used mechanical and thermal methods in rodents. Behav Brain Res. 2023; 446:114417. Doi: 10.1016/j.bbr.2023.114417
Inaltekin A, Kivrak Y. Evaluation of the effect of vortioxetine on pain threshold by hot-plate test in mice. Arch Neuropsychiatry. 2021; 58(4):274.
Annamalai P, Thangam EB. Local and systemic profiles of inflammatory cytokines in carrageenan-induced paw inflammation in rats. Immunol Invest. 2017; 46(3):274–283. https://doi.org/10.1080/08820139.2016.1248562
Falodun A, Okunrobo LO, Uzoamaka N. Phytochemical screening and anti-inflammatory evaluation of methanolic and aqueous extracts of Euphorbia heterophylla Linn (Euphorbiaceae). Afri J of Biotech. 2006; 5(6):529-531.
Regan RD, Fenyk-Melody JE, Tran SM, Chen G, Stocking KL. Comparison of submental blood collection with the retroorbital and submandibular methods in mice (Mus musculus). J Am Assoc Lab Anim Sci. 2016; 55(5):570–576.
Charrad R, Berraïes A, Hamdi B, Ammar J, Hamzaoui K, Hamzaoui A. Anti-inflammatory activity of IL-37 in asthmatic children: Correlation with inflammatory cytokines TNF-α, IL-β, IL-6 and IL-17A. Immunobiology. 2016; 221(2):182–187. Doi: 10.1016/j.imbio.2015.09.009
Roy A. Estimation of C-Reactive Protein Levels in Chronic Periodontitis. Indian J Public Health Res Dev. 2019; 10(6):1 Doi: 10.5958/0976-5506.2019.01228.2
Vishwakarma RK, Negi DS. The development of COX-1 and COX-2 inhibitors: a review. Int J Pharm Sci Res. 2020; 11(8):3544.
Weiss N, Zamponi GW. Opioid receptor regulation of neuronal voltage-gated calcium channels. Cell Mol Neurobiol. 2021; 41(5):839–847. Doi: 10.1007/s10571-020-00894-3
Yam MF, Loh YC, Tan CS, Khadijah Adam S, Abdul Manan N, Basir R. General pathways of pain sensation and the major neurotransmitters involved in pain regulation. Int J Mol Sci. 2018; 19(8):2164. Doi: 10.3390/ijms19082164
Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017; 2(1):1–9. Doi:10.1038/sigtrans.2017.23
Harvanová G, Duranková S, Bernasovská J. The role of cytokines and chemokines in the inflammatory response. Alergologia Pol. 2023; 10(3):210–219. Doi:10.5114/pja.2023.131708
Ronchetti S, Migliorati G, Delfino DV. Association of inflammatory mediators with pain perception. Biomed Pharmacother. 2017; 1(96):1445–1452. Doi:10.1016/j.biopha.2017.12.001
Vanderwall AG, Milligan ED. Cytokines in pain: harnessing endogenous anti-inflammatory signaling for improved pain management. Front Immunol. 2019; 23(10):3009. Doi:10.3389/fimmu.2019.03009
Petroianu GA, Aloum L, Adem A. Neuropathic pain: Mechanisms and therapeutic strategies. Front Cell Dev Biol. 2023; 11:1-17. Doi:10.3389/fcell.2023.1072629
Wang M, Thyagarajan B. Pain pathways and potential new targets for pain relief. Biotechnol Appl Biochem. 2022; 69(1):110–123. Doi:10.1002/bab.2086
Pereira-Leite C, Nunes C, Jamal SK, Cuccovia IM, Reis S. Nonsteroidal anti-inflammatory therapy: a journey toward safety. Med Res Rev. 2017; 37(4):802–859. Doi:10.1002/med.21424
Gunaydin C, Bilge SS. Effects of nonsteroidal anti-inflammatory drugs at the molecular level. Eurasian J Med. 2018; 50(2):116. Doi:10.5152/eurasianjmed.2018.0010
Germolec DR, Nunes C, Jamal SK, Cuccovia IM, Reis S. Markers of inflammation. Immunotoxicol Testing Methods Protoc. 2018; 57–79. Doi:10.1007/978-1-4939-8549-4_5
Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol. 2017; 13(7):399–409. Doi:10.1038/nrrheum.2017.83