Methanol Leaf Extract of Ricinus communis L. Mitigates Dichlorvos-Induced Cardiac Injury via Anti-Inflammatory, Antioxidant, and Anti-Apoptotic Mechanisms in Wistar Rats
Main Article Content
Abstract
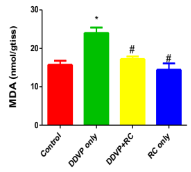
Dichlorvos induces cardiac injury via the activation of oxido-inflammatory signaling. This study investigates the cardioprotective potential of Ricinus communis methanol leaf extract (MERC) against dichlorvos-induced cardiac injury in the Wistar rat model. Thirty-two male Wistar rats were divided into four groups (A-D). Group A (control) received a vehicle solution (dimethyl sulfoxide and distilled water) for six weeks. Group B (DDVP only) inhalation exposure to dichlorvos occurred over a period of three weeks, during which no treatment was administered. Group C (DDVP + MERC) was exposed to dichlorvos and treated with MERC (300 mg/kg/day) orally for six weeks. Group D (MERC only) received MERC for six weeks. Cardiac biomarkers (lactate dehydrogenase (LDH), troponin-I, creatine kinase (CK-MB)), oxidative stress marker (malondialdehyde, MDA), antioxidant enzymes (SOD, CAT) and inflammatory markers (IL-6, MPO, TNF-α) were assessed alongside histological analysis. Dichlorvos exposure significantly increased CK-MB, LDH, troponin-I, IL-6, MPO, TNF-α, and MDA, while reducing SOD and CAT levels, indicating oxidative damage. Histology revealed myocardial degeneration, fibrosis, and inflammation. MERC treatment demonstrated dose-dependent protection, with optimal effects at 400 mg/kg. MERC reduced MDA levels, restored antioxidant enzyme activity, normalized cardiac biomarkers, and preserved heart tissue structure by reducing inflammation and fibrosis. These findings suggest MERC’s cardioprotective potential, owing to its dual capacity to reduce inflammation and combat oxidative stress. The study highlights Ricinus communis as a promising natural therapy for pesticide-induced cardiotoxicity.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25.
World Health Organization (WHO). Global action plan for the prevention and control of cardiovascular diseases. Geneva: WHO; 2018.
Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010;35:72–115.
Teo KK, Dokainish H. The emerging epidemic of cardiovascular risk factors and atherosclerotic disease in developing countries. Can J Cardiol. 2017;33(3):358–65.
Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O'Donnell M, Sullivan R. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385(9967):549–62.
Corsi DJ, Subramanian SV, Chow CK. Prospective Urban Rural Epidemiology (PURE) study: Baseline characteristics of the household sample and comparative analyses with national data in 17 countries. Am Heart J. 2013;166(4):636–46.
Murray CJL, Aravkin AY, Zheng P. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–49. https://doi.org/10.1016/S0140-6736(20)30752-2
Mohrman DE, Heller LJ, editors. Cardiovascular physiology. 5th ed. New York: McGraw-Hill; 2003.
Anand S, Singh S, Nahar Saikia U, Bhalla A, Paul Sharma Y, Singh D. Cardiac abnormalities in acute organophosphate poisoning. Clin Toxicol (Phila). 2009;47(3):230–5. https://doi.org/10.1080/15563650902724813
Forreira JP. Cardiovascular and non-cardiovascular death distinction: The utility of troponin beyond N-terminal pro-B-type natriuretic peptide. Findings from the BIOSTAT-CHF study. Eur J Heart Fail. 2020;22(1):81–9.
United States Environmental Protection Agency (USEPA). Dichlorvos TEACH chemical summary. Toxicity and exposure assessment for children. Washington, DC: USEPA; 2007.
Okoroiwu HU, Iwara IA. Dichlorvos toxicity: A public health perspective. Interdiscip Toxicol. 2018;11(2):129–37. https://doi.org/10.2478/intox-2018-0009
Saka WA, Igbayilola YD, Lawan JH, Muftaudeen TK, Adejumo R, Alu TD, et al. L-arginine supplementation mitigates dichlorvos-induced haematocardiotoxicity and oxidative stress in male Wistar rats. Open J Appl Sci. 2024;14:1886–903. https://doi.org/10.4236/ojapps.2024.147123
Saka WA, Adeogun AE, Adisa VI, Olayioye A, Igbayilola YD, Akhigbe RE. L-arginine attenuates dichlorvos-induced testicular toxicity in male Wistar rats by suppressing oxidative stress-dependent activation of caspase 3-mediated apoptosis. Biomed Pharmacother. 2024;178:117136. https://doi.org/10.1016/j.biopha.2024.117136
Saka WA, Igbayilola YD, Dada KO, Grema MG. The impact of L-arginine supplementation on thyroid function in male Wistar rats exposed to dichlorvos. Trends Med Res. 2024;19(1):151–9. https://doi.org/10.3923/tmr.2024.151.159
Saka WA, Igbayilola YD, Lawan HJ, Zakari MB, Awujoola DE, Olarinde PO, Adegoke VO. L-arginine supplement ameliorates dichlorvos-induced systemic inflammatory response and liver dysfunction in male Wistar rats. Toxicology Reports. 2024;101846. doi:10.1016/j.toxrep.2024.101846.
American Vanguard Corporation (AMVAC) Chemical Corp. Material Safety Data Sheet: DDVP Technical Grade. Los Angeles, CA: AMVAC; 1990.
Mostafalou S, Karami M, Mohammed A. Environmental and population studies concerning exposure to pesticides in Iran: A comprehensive review. Iran Red Crescent Med J. 2013;15(12):e13896.
Karki P, Ansari JA, Bhandary S, Koirala S. Cardiac and electrocardiographical manifestations of acute organophosphate poisoning. Singapore Med J. 2004; 45:385–9.
Henshaw UO, Iwara AI. Dichlorvos toxicity: A public health perspective. Interdiscip Toxicol. 2018;11(2):129–37.
Greim H, Saltmiras D, Mostert V, Strupp C. Evaluation of carcinogenic potential of the herbicide glyphosate, drawing on tumor incidence data from fourteen chronic/carcinogenicity rodent studies. Crit Rev Toxicol. 2015;45(3):185–208.
Bhinder P, Chaudhry A. Mutagenicity assessment of organophosphates using polymerase chain reaction-restriction fragment length polymorphism assay. Int Toxicol. 2013; 20:254–60.
Sobolev VE, Sokolova MO, Jenkins RO, Goncharov NV. Nephrotoxic effects of paraoxon in three rat models of acute intoxication. Int J Mol Sci. 2021;22(24):13625. doi:10.3390/ijms222413625.
Ahmed D, Khan MM, Saeed R. Comparative analysis of phenolics, flavonoids, and antioxidant and antibacterial potential of methanolic, hexanic and aqueous extracts from Adiantum caudatum leaves. Antioxidants (Basel). 2015;4(2):394–409. doi:10.3390/antiox4020394.
Alugah CI, Ibraheem O. Whole plant screenings for flavonoids and tannins contents in castor plant (Ricinus communis L.) and evaluation of their biological activities. Int J Herb Med. 2014; 2:68–76.
Elkordy AA, Haj-Ahmad RR, Amani SA. An overview on natural product drug formulations from conventional medicines to nanomedicines: Past, present, and future. J Drug Deliv Sci Technol. 2021; 63:102459. doi:10.1016/j.jddst.2021.102459.
Hitendra SC, Gayatri S, Babita J. Medicinal properties of Ricinus communis: A review. Int J Pharm Sci Res. 2021;12(7):3632–42. doi:10.13040/IJPSR.0975-8232.12(7).3632-42.
Altemimi A, Lakhssassi N, Baharlouei A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6(4):42. doi:10.3390/plants6040042.
Saka WA, Adisa VI, Igbayilola YD, Olayioye A, Adeogun AE. Hypoglycaemic, insulin modulation, and antioxidant potentials of Ricinus communis L. extracts in streptozotocin-induced diabetic male Wistar rats. Res J Med Plants. 2024;18(1):41–54. doi:10.3923/rjmp.2024.41.54.
Igbayilola YD, Morakinyo AO, Iranloye BO. Adverse effects of perinatal protein restriction on regulators of lipid metabolism and hepatic function in offspring of Sprague-Dawley rats. Niger J Exp Clin Biosci. 2021;9(2):74–81. doi:10.4103/njecp.njecp_49_20
Igbayilola YD, Morakinyo AO, Iranloye BO. Adverse effects of perinatal protein restriction on glucose homeostasis in offspring of Sprague-Dawley rats. Sci Afr. 2021; 14:1-11. https://doi.org/10/j.sciaf/e01036.
Igbayilola YD, Aina OS, Mofolorunso AM, Ashiru MA. Hypolipidaemic, antioxidant and hepatoprotective effects of cucumber (Cucumis sativus L.) supplemented diet in both sexes of Sprague-Dawley rats. Niger J Exp Clin Biosci. 2021;9(2):82-8. https://doi.org/10.4103/njecp.njecp_1_21.
Igbayilola YD, Saka WA, Aina OS, Mofolorunso AM, Ashiru MA. Antihyperlipidemic and antioxidant potentials of aqueous leaf extract of Telfairia occidentalis (fluted pumpkin) in male offspring of Sprague-Dawley rats. Niger J Physiol Sci. 2021;36:109-14.
Igbayilola YD, Morakinyo AO, Iranloye BO. Leptin-resistance induced hyperphagia and diminished oxidative balance in offspring of dams exposed to perinatal protein restriction. Afr J Biomed Res. 2021;24:451-458.
Igbayilola YD, Gujja MG. Alpha-amylase and alpha-glucosidase upregulated glucose homeostasis in high-fat fed Wistar rats supplemented with cocoa flavonoid-rich aqueous extract. Food Biosci. 2024;104070. https://doi.org/10.1016/j.fbio.2024.104070.
American Veterinary Medical Association. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Schaumburg, IL: AVMA; 2020.
Igbayilola YD, Grema MG, Jibrin S. Molecular docking assessment of cocoa bean flavonoid extract and its impact on lipase enzymes and blood markers in high-fat diet fed rats. Food Hum. 2024;100443. https://doi.org/10.1016/j.foohum.2024.100443.
Afolabi OA, Anyogu DC, Hamed MA, Odetayo AF, Adeyemi DH. Glutamine prevents upregulation of NF-kB signaling and caspase 3 activation in ischaemia/reperfusion-induced testicular damage: An animal model. Biomed Pharmacother. 2022; 150:113056. https://doi.org/10.1016/j.biopha.2022.113056.
Roth EF Jr, Gilbert HS. The pyrogallol assay for superoxide dismutase: Absence of a glutathione artifact. Anal Biochem. 1984;137(1):50-3. https://doi.org/10.1016/0003-2697(84)90344-0.
Iwase T, Tajima A, Sugimoto S, Okuda K, Hironaka I, Kamata Y, et al. A simple assay for measuring catalase activity: A visual approach. Sci Rep. 2013; 3:3081. https://doi.org/10.1038/srep03081.
Appala RN, Chigurupati S, Appala RV, Selvarajan KK, Mohammad IJ. A simple HPLC-UV method for the determination of glutathione in PC-12 cells. Scientifica. 2016; 2016:6897890. https://doi.org/10.1155/2016/6897890.
Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem. 2017; 524:13-30. https://doi.org/10.1016/j.ab.2016.10.021.
Ruyssers NE, De Winter BY, De Man JG, Loukas A, Pearson MS, Weinstock JV, et al. Therapeutic potential of helminth soluble proteins in TNBS-induced colitis in mice. Inflamm Bowel Dis. 2009;15(4):491-500.
Mohamed AK, Magdy M. Caspase 3 role and immunohistochemical expression in assessment of apoptosis as a feature of H1N1 vaccine-caused drug-induced liver injury (DILI). Electron Physician. 2017;9(5):4261-73. https://doi.org/10.19082/4261.
Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta. 2006;1757(5-6):639-47. https://doi.org/10.1016/j.bbabio.2006.03.016.Here are the references formatted in Vancouver style:
Guzy PM. Creatine phosphokinase-MB (CPK-MB) and the diagnosis of myocardial infarction. West J Med. 1977;127(6):455–60.
Parsanathan R, Jain SK. Novel invasive and noninvasive cardiac-specific biomarkers in obesity and cardiovascular diseases. Metab Syndr Relat Disord. 2020;18(1):10–30. https://doi.org/10.1089/met.2019.0073
Al-Hadi HA, Fox KA. Cardiac markers in the early diagnosis and management of patients with acute coronary syndrome. Sultan Qaboos Univ Med J. 2009;9(3):231–246.
Morakinyo AE, Omoniyi FE, Nzekwe SC, Oyebamiji AK, Adelowo JM, Lawal SA, Olumade AA, Olopade EO, Oyedepo TA. Cardio-protective effect Hunteria umbellate seed: Experimental and in silico approaches. Trop J Nat Prod Res. 2022; 6 (1): 87-94. https://tjnpr.org/index.php/home/article/view/200
Babuin L, Jaffe AS. Troponin: the biomarker of choice for the detection of cardiac injury. CMAJ. 2005;173(10):1191–202. https://doi.org/10.1503/cmaj/051291
Skeik N, Patel DC. A review of troponins in ischemic heart disease and other conditions. Int J Angiol. 2007;16(2):53–58. https://doi.org/10.1055/s-0031-1278248
Klein R, Nagy O, Tóthová C, Chovanová F. Clinical and diagnostic significance of lactate dehydrogenase and its isoenzymes in animals. Vet Med Int.2020:5346483. https://doi.org/10.1155/2020/5346483
Syahputra RA, Harahap U, Dalimunthe A, Nasution MP, Satria D. The role of flavonoids as a cardioprotective strategy against doxorubicin-induced cardiotoxicity: A review. Molecules. 2022;27(4):1320. https://doi.org/10.3390/molecules27041320
Shargorodsky M, Debby O, Matas Z, Zimlichman R. Effect of long-term treatment with antioxidants (vitamin C, vitamin E, coenzyme Q10, and selenium) on arterial compliance, humoral factors, and inflammatory markers in patients with multiple cardiovascular risk factors. Nutr Metab. 2010;7:55. https://doi.org/10.1186/1743-7075-7-55
Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74(3):417–33. https://doi.org/10.1128/MMBR.00016-10
Tracey KJ, Wei H, Manogue KR, Fong Y, Hesse DG, Nguyen HT, et al. Cachetin/tumor necrosis factor induces cachexia, anemia, and inflammation. J Exp Med. 1988;167(4):1211–27.
Muthusamy A, Jayabalan N. Effect of factory effluents on physiological and biochemical contents of Gossypium hirsutum. J Environ Biol. 2001;24(4):237–42.
Imam A, Busari M, Adana M, Ajibola M, Ibrahim A, Sulaiman F, et al. Subchronic dichlorvos-induced cardiotoxicity in Wistar rats: Mitigative efficacy of Nigella sativa oil. J Exp Clin Anat. 2018;17(2):1330.
Ilavarasan R, Mallika M, Venkataraman S. Anti-inflammatory and free radical scavenging activity of Ricinus communis root extract. J Ethnopharmacol. 2006;103(3):478–80. https://doi.org/10.1016/j.jep.2005.07.029
Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as a toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15(4):316–28. https://doi.org/10.1016/j.numecd.2005.05.003
Akomolafe SF, Kehinde AJ, Ajayi OB, Olofinniyi JA, Olasehinde TA. Assessment of cashew (Anacardium occidentale L.) nut supplemented diet on key biochemical indices relevant to cardiac function in cisplatin-induced cardiotoxic rats. Trop J Nat Prod Res. 2024; 8 (3): 6705-6712. http://doi.org/10.26538/tjnpr/v8i3.34
Saka WA, Adisa VI, Igbayilola YD, Olayioye A, Adeogun AE. Hypoglycaemic, insulin modulation and antioxidant potentials of Ricinus communis L. extracts in streptozotocin-induced diabetes in male Wistar rats. Res J Med Plants. 2024;18(1):41–54. https://doi.org/10.3923/rjmp.2024.41.54
Zhang W, Zheng Y, Yan F, Dong M, Ren Y. Research progress of quercetin in cardiovascular disease. Front Cardiovasc Med. 2023; 10:1203713. https://doi.org/10.3389/fcvm.2023.1203713
Perez-Garijo FA, Martin G, Struhl G, Morata D. Signaling and the induction of neoplastic tumors by caspase-inhibited apoptotic cells in Drosophila. Proc Natl Acad Sci U S A. 2005;102(50):17664–17669.
Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495-516. doi:10.1080/01926230701320337